Brain Network Connectivity and Association with Catechol-O-Methyltransferase Gene Polymorphism in Korean Attention-Deficit Hyperactivity Disorder Children
Article information
Abstract
Objective
We sought to determine if the links between and within the default mode network (DMN) and dorsal attention network (DAT) exhibited different conditions according to catechol-O-methyltransferase (COMT) gene polymorphism in relationship to attention-deficit hyperactivity disorder (ADHD) symptoms.
Methods
Fifty-seven children with ADHD and 48 healthy controls (HCs) were administered an intelligence test, the Children’s Depression Inventory, the Korean ADHD rating scale, and continuous performance test. Resting-state brain functional MRI scans were obtained, and COMT genotyping was performed to distinguish valine carriers and methionine homozygotes.
Results
Compared to controls, children with ADHD showed increased ADHD scale scores, increased visual commission errors, and increased functional connectivity (FC) within the DMN and DAT. Compared to all children with ADHD, children with the methionine homozygote and those who were valine carriers showed increased FC within the DMN and DAT and decreased FC between the DMN and DAT. FC within the DMN was also increased in HC valine carriers compared to HC children with the methionine homozygote, and in children with ADHD who were valine carriers compared to HC valine carriers.
Conclusion
We observed increased brain connectivity within the DMN and DAT and altered brain connectivity within and between the DMN and DAT associated with COMT polymorphism in children with ADHD.
INTRODUCTION
Attention-deficit hyperactivity disorder (ADHD) is a neurodevelopmental disorder characterized by inattention, impulsivity, and hyperactivity and has a worldwide prevalence of 5.3% [1,2]. Although several genetic and environmental studies have attempted to understand the pathogenesis of ADHD [3,4], its exact etiology and pathogenesis are unknown and is thought to be a complex, polygenic disorder [5]. Specifically, the heritability of ADHD is estimated to be about 80% according to a meta-analysis. Clinical studies [6,7] have demonstrated abnormalities of the dopaminergic, noradrenergic system and frontal-striatal brain systems [8,9] associated with ADHD.
Many genetic and neuroimaging approaches have been actively studied to clarify the pathogenesis of ADHD mentioned above. Through functional magnetic resonance imaging (fMRI) studies, the core brain regions involved in attention control, and their connectivity and activation levels have been studied. These consist of the dorsal and ventral lateral frontal cortices and the posterior parietal area [10-12]. These regions form an attentional control system consisting of the dorsal and ventral attention networks (DAT and VAT) which operate as an integrated supramodal top-down and bottom-up attentional gating system [10,13]. While traditional accounts of attentional function and dysfunction have focused on task-dependent neural activity within these networks, recent formulations have stressed the importance of a task-independent network [14]. This network, termed the default mode network (DMN), is a large and robustly replicable network of brain regions that are associated with task-irrelevant mental processes. These consist of frontal and posterior midline structures (the medial prefrontal cortex and posterior cingulate cortex with adjacent precuneus) and lateral parietal and medial temporal lobe regions [15]. The DMN shows higher activity and stronger functional connectivity (FC) during rest, and its activity is attenuated following the onset of tasks [15]. Persistence of DMN activity during tasks has been shown to predict errors in task output, and unsuccessful attenuation of the DMN is reportedly associated with momentary lapses in attention denoted by longer reaction times and less accurate performance in an attentional control task [16,17]. When one considers the data from these studies, it seems that effective attentional engagement requires both the “switching on” of the task-positive attention networks and the “switching off” of the DMN [17-19]. Actually, many recent studies showed evidence of abnormal coordination of the DMN and attention networks, adversely affecting performance in individuals with ADHD [20-24].
Several genetic studies, and brain imaging studies, have been carried out to determine the etiology of ADHD. In particular, imbalance and dysregulation of the dopaminergic and noradrenergic neurotransmitter systems of the central nervous system are key mechanisms related to ADHD [8]. Many genetic studies related to this activation and catabolism have been carried out. Catechol-O-methyltransferase (COMT) is an important enzyme that plays a major role in the degradation of catecholamine, including dopamine (DA) and norepinephrine (NE), in the synapses [25]. As a result, excessive activation of COMT enzymes lowers the activity of DA and NE in the attention-related brain networks, resulting in clinical impairment of this executive function. These effects are closely related to the symptom expression of ADHD, so the COMT gene is an essential candidate gene for the etiology of ADHD [26,27]. A single nucleotide polymorphism (SNP, rs4680) in COMT is the most popular SNP in ADHD, leading to valine (Val) to methionine (Met) substitution at codon 158 (Val158-Met) of the COMT. This induces an approximately three- to four-fold decrease in enzyme activity, resulting in increased catecholamine activity [28-30]. There is considerable evidence that the COMT Val/Met SNP is highly related to ADHD, and in a recent Korean study [31], it was revealed that the COMT Val allele of Val158-Met polymorphism is associated with ADHD within the Korean population [32]. Also, children with ADHD with the Val homozygote demonstrated a good response to methylphenidate treatment [33].
We think that it is important to clarify the relationship between the brain imaging findings and the genetic results of ADHD mentioned above and integrate the neuro-genetic and neuroimaging findings together to take a step closer to the pathogenesis of ADHD. However, few studies have mentioned the association between COMT polymorphism and brain FC [34,35], and no study has directly investigated the relation between COMT polymorphism and brain DMN or between COMT polymorphism and the attention network in ADHD.
We hypothesized that, in children with ADHD, the FC within the DMN and DAT was decreased compared to healthy controls (HCs). Additionally, we believed that children with ADHD who were Val carriers would show increased FC within the DMN and DAT, compared to children with ADHD with the Met homozygote.
METHODS
Participants
Initially, 60 children with ADHD and 50 age- and sex-matched HCs were recruited through the psychiatry department at Chung Ang University Hospital. Of the 60 children with ADHD, two had intelligence quotients (IQs) lower than 80. One patient with ADHD and one control participant could not be scanned in an MRI scanner due to claustrophobia. One HC participant showed depressive symptoms. Finally, 57 patients with ADHD and 48 HCs completed the study protocol.
All study procedures and protocols were explained to the patients and controls and their parents. Informed consent was obtained from the patients and controls, and written informed consent was obtained from their parents. The protocol for the current study was approved by the Institutional Review Board of Chung Ang University Hospital [C2012033 (728)].
All participants in the current study were assessed with the Korean Kiddie Schedule for Affective Disorders and Schizophrenia-Present and Lifetime version (K-SADS-PL) [36], and diagnosed by a child and adolescent psychiatric doctor (DHH) following a clinical interview. IQ, depression severity, and ADHD symptoms were assessed using the Korean-Wechsler intelligence scale for children (K-WISC-IV) [37], the Children’s Depression Inventory (CDI) [38], and the Korean ADHD rating scale (K-ARS) for parents, respectively [39]. Attention was assessed using a standardized version of continuous performance test (CPT) for Korean children and adolescents, and its reliability and validity as a diagnostic instrument for ADHD has been established [40,41]. The Korean version of the CPT consists of visual and auditory attention tests, each of which takes 15 minutes to complete. The CPT test results included omission errors, commission errors, response time mean, and response time deviation. In this study, we used two major variables: omission errors (a measure of inattention) and commission errors (a measure of impulsivity) in CPT. The exclusion criteria were: 1) a history of another axis I psychiatric disease except for ADHD, 2) IQ <80, and 3) a history of neurological or medical disorders.
Imaging processing and analysis
The children with ADHD underwent a 7-day medication washout period before their study enrollment and fMRI scans. For the brain connectivity analyses, all children with ADHD and HCs completed a resting-state fMRI (rs-fMRI) study using an MRI scanner (Philips Achieva 3.0 Tesla TX MRI scanner). The scanning parameters were as follows: TR=3 s, 12-minute scan, 240 volumes, 128×128 matrix, 40 slices at 4.0 mm slice thickness. Preprocessing included despiking (AFNI: 3dDespike), motion correction, coregistration to MPRAGE image, normalization to MNI space in SPM 12b, temporal detrend (Matlab: detrend.m), bandpass filtering (Matlab: idealfilter.m), and voxelwise regression of identically bandpass filtered time series of six head motion parameters.
Children with ADHD and HCs were asked to remain awake with their eyes closed. To avoid head movements, the children’s heads were stabilized with cushions. To avoid microhead movements, we applied realignment steps using six rigidbody parameters with each participant’s estimated motion [42-44], but no regression of the global signal was performed.
We extracted eight regions of two brain networks (four DMNs: the middle prefrontal cortex, right/left lateral parietal cortex, posterior cingulate cortex; four dorsal attention networks: right/left frontal eye field, right/left inferior parietal sulcus) from the Automated Anatomical Labeling (AAL) atlas of the brain (AAL ver 2) [45], which were found in group independent component analysis (ICA) analysis of all participants [46]. Fisher-transformed correlation coefficients were measured for each pair of ROIs in each participant. We calculated the FC between regions of interest (ROIs) using the CONN-fMRI Functional Connectivity Toolbox (ver.15; www. Nitrc.org/projects/conn). Between-group effects were considered significant with a cluster-level false discovery rate (FDR) and p values less than 0.05.
Genotyping
Genotyping was performed at the Laboratory of Labgenomics, Korea. According to the manufacturer’s protocol, genetic DNA was extracted from blood (stored frozen) using a G-DEXTM II Genomic DNA Extraction Kit (Intron Biotechnology, Seoul, Korea). The SNPs were analyzed using the polymerase chain reaction-ligase detection reaction (PCR-LDR) method. First, the PCR reaction was performed in a volume of 20 µL containing PCR master mix (Nanohelix, Korea), 500 nM of each primer of Table 1, and about 50 ng genomic DNA. The reaction consisted of denaturation at 95°C for 15 min, followed by 40 cycles of 95°C for 20 s, 55°C for 40 s, and 72°C for 1 min, with a final extension at 72°C for 5 min. Following PCR, unincorporated primers and dNTPs were removed by adding 1/10 volume of Exo-Sap (ExoProStar 1, GE Healthcare) and incubating for 15 min at 37°C, followed by 15 min at 85°C for enzyme inactivation. Then, the LDR reaction was carried out in a buffer containing 4 µL of PCR product, 10X ligase buffer (NEB), 12.5 nM each allele-specific probe (left probe, Bioneer, Korea) and 25 nM each common probe (right probe, Bioneer, Korea) of Table 1, and 1.25 units of 9° NTM ligase (NEB). The reaction volume was 20 µL, and the LDR consisted of 25 cycles of 94°C for 60 seconds and 65 °C for 150 seconds. The LDR products were then analyzed by ABI 3730 Gene Analyzer (Applied Biosystems).
RESULTS
Demographic data
There was a significant difference in K-ARS scores and visual commission errors between children with ADHD and HCs. However, there were no significant between-group differences in age, education year, CDI scores, and COMT gene distribution (Table 2).
Of the 57 children with ADHD, 23 children were of the inattentive type, 21 were combined type, and 13 were hyperactive type. All children with ADHD took methylphenidate 27.9±13.2 mg/day.
Finding the best-matched network for our data
During the group ICA analysis of the 105 participants, five brain circuits including the DMN, sensory-motor (SM), visual (VS), DAT, and cerebellar network were best-matched (Figure 1). Of the five regions, we selected two networks (DMN and DAT), which were already reportedly associated with children with ADHD in previous studies (Figure 1).
Comparisons of FC between children with ADHD and HCs
Compared to HCs, children with ADHD showed increased FC within the DMN (left lateral parietal-right lateral parietal, left lateral parietal-posterior cingulate gyrus, right lateral parietal-posterior cingulate gyrus, middle prefrontal gyrus-posterior cingulate gyrus, middle prefrontal gyrus-right lateral parietal, middle prefrontal gyrus-left lateral parietal) and DAT (left inferior parietal sulcus-right inferior parietal sulcus, right frontal eye field-right inferior parietal sulcus, left frontal eye field-right frontal eye field, left frontal eye field-left inferior parietal sulcus, left frontal eye field-right inferior parietal sulcus) (Figure 2, Table 3).
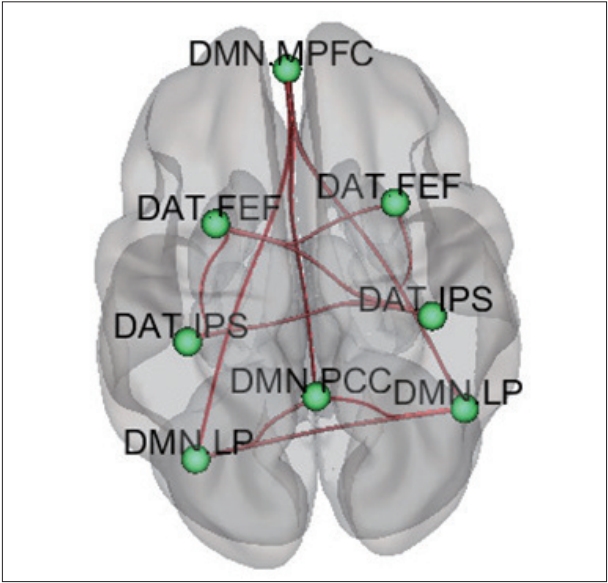
Comparison of functional connectivity between ADHD children and healthy children. DMN: Default mode network, DAT: Dorsal attention network, MPFC: middle prefrontal cortex, FEF: frontal eye field, IPS: inferior parietal sulcus, LP: lateral parietal lobe, PCC: posterior cingulate cortexDMN: Default mode network, DAT: Dorsal attention network, MPFC: middle prefrontal cortex, FEF: frontal eye field, IPS: inferior parietal sulcus, LP: lateral parietal lobe, PCC: posterior cingulate cortex.
Correlations between FC within brain networks and psychological test results
Visual commission errors in children with ADHD were associated with FC within the DMN (left lateral parietal-right lateral parietal). There were no significant differences in visual commission errors between children with ADHD with Val carriers and children with ADHD with Met homozygote.
Comparison of FC between children with ADHD with the Met homozygote and children with ADHD who were Val carriers
Compared to children with ADHD with the Met homozygote (n=24), children with ADHD who were Val carriers (n=33) showed increased FC within the DMN (left lateral parietal-posterior cingulate, left lateral parietal-right lateral parietal) and DAT (right frontal eye filed-right inferior parietal sulcus).
Compared to children with ADHD with the Met homozygote (n=24), children with ADHD who were Val carriers (n=33) showed decreased FC between the DMN and DAT (posterior cingulate-right frontal eye field, right lateral parietal-left inferior parietal sulcus) (Figure 3, Table 3).
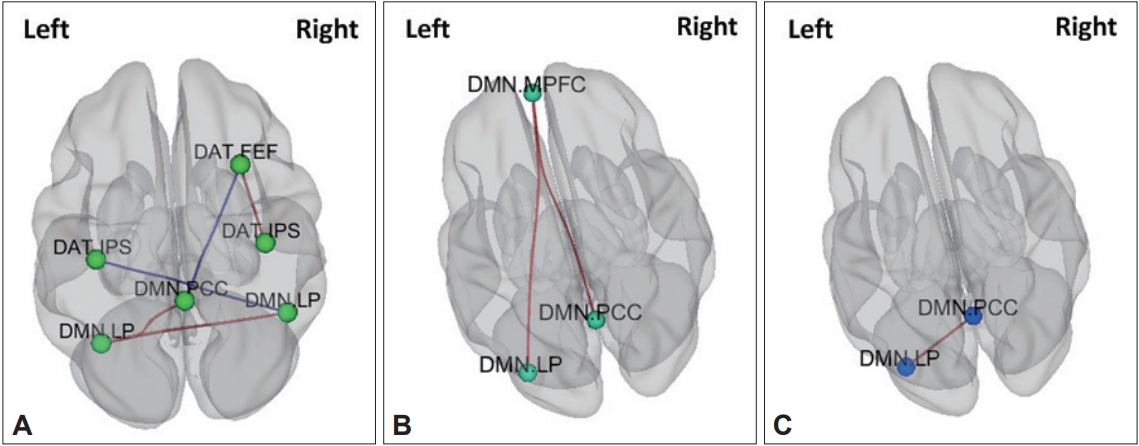
Genetic neuroimaging results in patients with ADHD and healthy comparison children. A: Comparison of functional connectivity between ADHD children with Val carriers and ADHD children with Met homozygote, B: Comparison of functional connectivity between healthy comparison (HC) children with Val carriers and HC children with Met homozygote, C: Comparison of functional connectivity between ADHD children with Val carriers and HC children with Val carriers. DMN: Default mode network, DAT: Dorsal attention network, MPFC: middle prefrontal cortex, FEF: frontal eye field, IPS: inferior parietal sulcus, LP: lateral parietal lobe, PCC: posterior cingulate cortex.
Comparison of FC between healthy children with Met homozygote and healthy children with Val carriers
Compared to HCs with the Met homozygote (n=25), HC children who were Val carriers (n=23) showed increased FC within the DMN (middle prefrontal cortex-posterior cingulate, the middle prefrontal cortex-left lateral parietal) (Figure 3, Table 3).
Comparison of FC between children with ADHD with Val carriers and healthy children with Val carriers
Compared to HC children who were Val carriers (n=33), children with ADHD who were Val carriers (n=23) showed increased FC within the DMN (posterior cingulate-left lateral parietal) (Figure 3, Table 3).
DISCUSSION
We analyzed the FC within the DMN and DAT networks and between the DMN and DAT networks according to genetic COMT subtype (Val carriers vs. Met homozygotes) in children with ADHD and HCs. In this way, the relationship between brain attentional control (the DMN and DAT) and one of the core candidate genes associated with ADHD (COMT Val158-Met polymorphism) could be examined.
Comparisons of FC between children with ADHD and HCs
We found hyperconnectivity within the DMN as well as within the DAT in children with ADHD compared to HCs in the resting-state. These findings were similar to the results of other ADHD studies [47-49]. Sidlauskaite et al. [48] reported that the connectivity within DMN regions—representing the task-independent brain activity state like resting status—was more imbalanced in individuals with ADHD compared to HCs. Moreover, high FC within the DMN in the resting-state is considered ineffective for rapid switching of the brain to the concentration state when a sudden stimulus or goal-related task is given [47]. It becomes difficult to exert effective quality attention, resulting in the clinical symptoms that characterize ADHD [19,23,48].
We also found hyperconnectivity within DAT in children with ADHD, which was consistent with another recent study [48]. Usually, the DAT and VAT systems are differentiated by their specific functions in attentional control [13]. Also, the interplay between these systems during attentional processing is important for sustained elaborate attention [50]. However, the increased connectivity within the DAT may lead to dysfunction of the integrated attention [50]. This means that there might be a problem with efficient information exchange and complementary function between the DAT and VAT, potentially leading to incomplete cognitive and attentional functions when a task is given.
However, other studies of ADHD produced controversial results. Differences in fMRI analytic method techniques among previous studies could explain the inconsistent DMN connectivity findings. In the same participants, the one seed-based analytic study and the other network homogeneity analytic study appeared to show decreased connectivity within DMN regions [51,52]. Readers should consider controversial results with various analytic methods when interpreting the findings.
Correlations between FC within brain networks and psychological test results
Interestingly, CPT test visual commission errors in the current study were significantly higher in the ADHD group than in the HCs, and visual commission errors showed a significant positive correlation with DMN connectivity. Among the CPT test variables, commission errors, which are false responses, represent impulsivity and are well established neurocognitive endophenotypes of ADHD [53]. Several studies have suggested that the difficulties in impulse control were associated with increased FC within the DMN [54,55].
Comparison of FC between children with ADHD with the Met homozygote and children with ADHD who were Val carriers
The availability of DA and NE in the prefrontal cortex is mainly affected by COMT enzymes [56]. Recent research showed that altered dopaminergic and noradrenergic tone, affected by COMT genotypes including the Val158-Met polymorphism, resulted in neuroadaptive changes in several task-positive and negative network basal FC. This could, in turn, contribute to its effects on behavior [57-62]. Endogenous DA and NE have a large role in working memory performance, and both systems are inseparable for normal executive function [63].
In the current study, increased FC within the DMN and DAT might provide preliminary evidence of lowered DA and NE levels in these networks in the Val polymorphism compared to the Met. This finding appears to demonstrate a more severe attentional crisis in children with ADHD and the Val polymorphism, consistent with our findings comparing FC in children with ADHD to HCs. This is also supported by the fact that FC within the DMN was higher in the Val group than in the Met HC group. Interestingly, even with the same Val type, the ADHD Val group had a higher FC within the DMN than the HC Val group.
DA and NE levels may be decreased in those with the Val allele compared to those with the Met allele [28-30]. Also, the availability of DA and NE in the prefrontal cortex is mainly affected by COMT enzymes [56]. Recent research shows the altered DA and NE tone, affected by COMT genotypes, including the Val158-Met polymorphism, resulting in neuroadaptive changes in several task-positive and negative network basal FC. This could contribute to its effects on behavior [57-62]. Endogenous DA and NE have a large role in working memory performance, and both systems are inseparable for normal executive function [63].
The decreased FC finding “between” the DMN and DAT in children with the Val polymorphism—compared to the Met—was consistent with another research report on ADHD [64]. This finding was also mentioned as a distinct intrinsic network connective feature of the ADHD group compared to the HCs, suggesting a diminished antagonistic connection between the DMN and attention networks. This may result in excess task-related DMN connectivity [51,65,66].
Taking these findings together, ADHD symptoms may be associated with the polygenic etiologies of genetic and brain development. Also, these substantial findings in ADHD were more characteristic to the Val polymorphism than to the Met. Val carriers may exhibit more evident ADHD traits, suggesting that it is one of the candidate genes involved in the etiology of ADHD.
Limitations
Our research findings elucidate brain FC in children with ADHD and the relationship to DA-related gene effects. However, our study has some limitations. Due to the small number of participants, it would be insufficient to say that our findings were due to genetic differences, so future studies should analyze additional participants to boost study reliability. We are hopeful that future research efforts will continue to clarify default and attention-related network connectivity, considering ADHD subtypes as well as the effects of medication (methylphenidate). Second, the heterogeneity of the ADHD group in this study should be considered.
ADHD is a complex disorder, and its subtypes can be further sub-divided into three categories: inattentive, hyperactive, and combined [67]. We did not distinguish among the ADHD group subtypes, so future studies should clarify and account for these differences. Thirdly, we did not perform other neuropsychiatric tests that could assess executive function and attention, opting instead to only administer the CPT. Therefore, we were limited in our ability to evaluate various symptoms of ADHD and link these symptoms to neurophysiologic and genetic factors. Lastly, although the participants with ADHD had a sufficient medication washout period before their study enrollment and fMRI scan, we did not consider individual histories and durations of stimulant and other psychoactive medication use, such as antidepressants. Such medications may exert different effects on functional brain organization and should be considered by future studies.
In conclusions, we found increased brain connectivity within the DMN and DAT in children with ADHD. The COMT Val158-Met polymorphism was related to altered brain connectivity between the DMN and DAT due to its effect on DA and NE. These findings suggest that, in individuals with ADHD, there is disrupted connectivity between brain networks, particularly attention-related shifting networks, including the DMN. Moreover, these are a neurophysiological phenomenon of ADHD and dopaminergic and noradrenergic actions may affect the FC of these networks. These factors might be related to the pathophysiology of ADHD.
Acknowledgements
This study was supported by grants from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A120013) and Korea Creative Content Agency (R2014040055).
Notes
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Doug Hyun Han. Data curation: Jeong Ha Park. Formal analysis: Young Don Son. Funding acquisition: Doug Hyun Han, Yeni Kim. Investigation: Jeong Ha Park. Methodology: Young Don Son. Project administration: Doug Hyun Han. Resource: Doug Hyun Han. Software: Young Don Son. Supervision: Doug Hyun Han. Validation: Yeni Kim. Visualization: Jeong Ha Park. Writing—original draft: Jeong Ha Park. Writing—review & editing: Doug Hyun Han.




