Understanding the Complex of Suicide in Depression: from Research to Clinics
Article information
Abstract
Objective
Amongst psychiatric disorders, major depressive disorder (MDD) is the most prevalent, by affecting approximately 15–17% of the population and showing a high suicide risk rate equivalent to around 15%. The present comprehensive overview aims at evaluating main research studies in the field of MDD at suicide risk, by proposing as well as a schematic suicide risk stratification and useful flow-chart for planning suicide preventive and therapeutic interventions for clinicians.
Methods
A broad and comprehensive overview has been here conducted by using PubMed/Medline, combining the search strategy of free text terms and exploded MESH headings for the topics of ‘Major Depressive Disorder’ and ‘Suicide’ as following: ((suicide [Title/Abstract]) AND (major depressive disorder [Title/Abstract])). All articles published in English through May 31, 2019 were summarized in a comprehensive way.
Results
Despite possible pathophysiological factors which may explain the complexity of suicide in MDD, scientific evidence supposed the synergic role of genetics, exogenous and endogenous stressors (i.e., interpersonal, professional, financial, as well as psychiatric disorders), epigenetic, the hypothalamic-pituitary-adrenal stress-response system, the involvement of the monoaminergic neurotransmitter systems, particularly the serotonergic ones, the lipid profile, neuro-immunological biomarkers, the Brain-derived neurotrophic factor and other neuromodulators.
Conclusion
The present overview reported that suicide is a highly complex and multifaceted phenomenon in which a large plethora of mechanisms could be variable implicated, particularly amongst MDD subjects. Beyond these consideration, modern psychiatry needs a better interpretation of suicide risk with a more careful assessment of suicide risk stratification and planning of clinical and treatment interventions.
INTRODUCTION
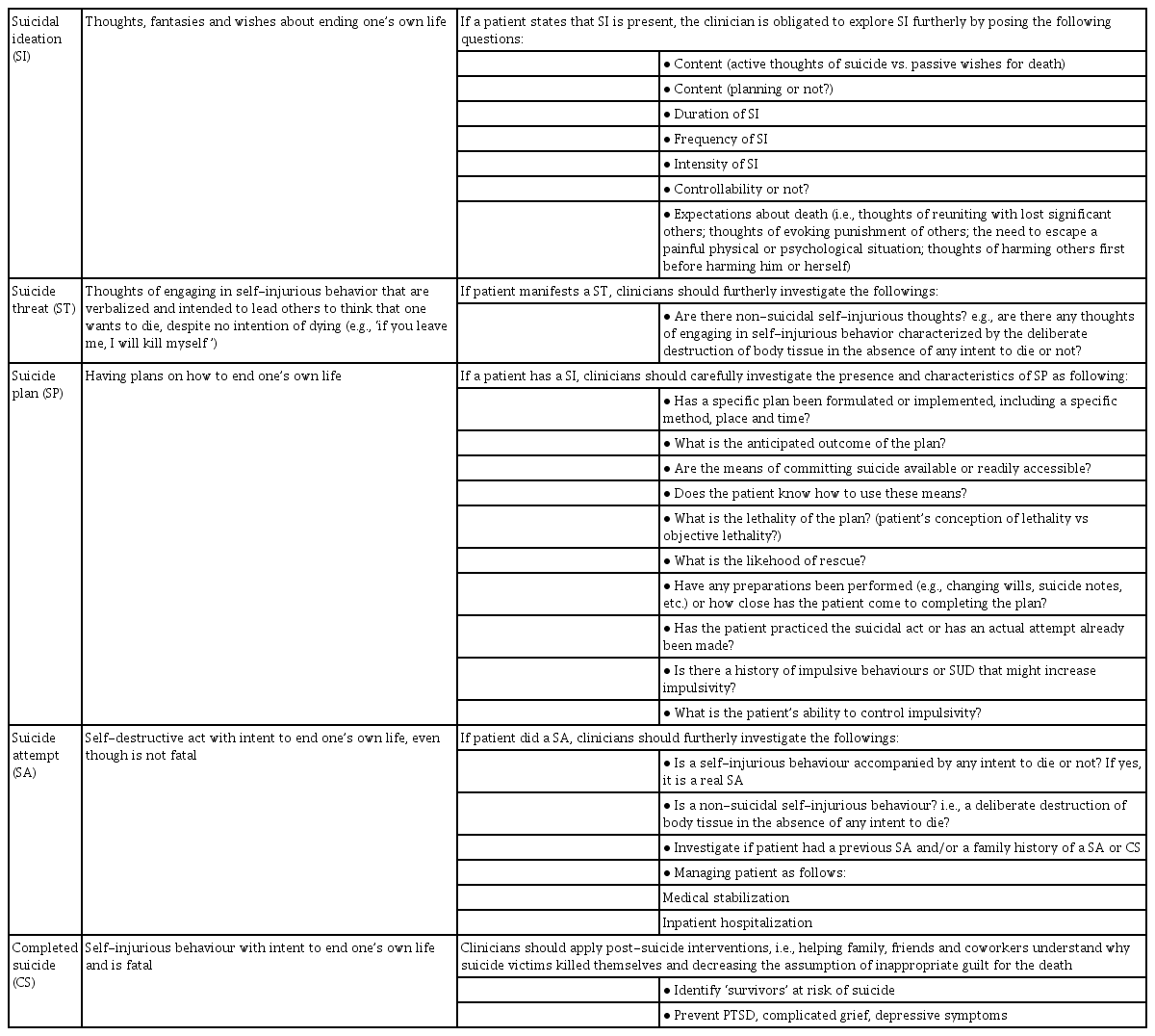
Suicide is a leading public health problem, being a leading cause of injury and death at a worldwide level, with approximately one million people who die by suicide per year and an estimate of around one suicide death occurring every 40 seconds [1-3]. Suicide is ranked as the 2th leading cause of death among people aged 10 to 34 and the tenth among all age groups [3,4]. Notably, suicidal behaviour has been implicated as a co-morbidity of several neuropsychiatric disorders, including borderline personality disorder, schizophrenia, bipolar disorder and major depressive disorder (MDD), being considered one of the leading causes of preventable death amongst people affected with mental disorders [5]. MDD is a common psychiatric disorder which is associated with significant personal suffering, physical and mental disability, with a global point prevalence being around 4.7% and a lifetime prevalence ranging from 3% in Japan to 16.9% in USA, whilst in other Western countries the figures varied between 8% and 17% [6-8]. The association between MDD and suicide attempts (SA) and/or ideation (SI) has been well documented, being SI and suicidal behaviour frequently reported during depressive episodes, with a suicide risk rate equivalent to around 15% [3,9-11]. Furthermore, epidemiological studies reported as well that MDD subjects with comorbid anxiety disorders were among the main predictors of SA amongst depressed suicide subjects [12]. However, the role of comorbid anxiety disorders, in increasing suicidal risk is still a matter of debate although it has been well recognized that the association between MDD and anxiety disorders appear to have more a synergic role in increasing suicidal risk [13]. Despite possible pathophysiological factors which may determine/ explain the correlation between depression and suicidal risk are not yet fully understood, it has been supposed the synergic role of genetics, exogenous and endogenous stressors (i.e., interpersonal, professional, financial, as well as psychiatric disorders), the hypothalamic-pituitary-adrenal (HPA)-stressresponse system, epigenetics, the involvement of the monoaminergic neurotransmitter systems, particularly the serotonergic ones, the role of specific neurotrophins, such as the Brain-derived neurotrophic factor (BDNF), etc [14-16]. Overall, suicide is a complex phenomenon and, according to the World Health Organization [3], suicidal behaviour may be defined as “a range of behaviours that include thinking about suicide (suicidal ideation, SI), suicide threat (ST), planning for suicide (SP), attempting suicide (SA) and suicide itself (CS)” (Table 1). The complex phenomenon is not due to a simple etiology but rather is the result of a complex interaction of genetic vulnerability, stress factors, underlying psychopathology and social aspects, even though the precise pathophysiological mechanisms underlining the suicide behaviour is not yet fully understood and probably not completely investigated. Being literature published so far on suicide risk within patients affected with MDD extremely varied and broad, the present paper aimed at overviewing only a selected range of suicide risk predictors in MDD subjects, by analysing both research and clinical evidence, as primary objective, to try identifying a pathophysiological as well as clinical perspective, able to answer to questions regarding performing preventive tools (if any), easy to measure and useful for clinical practice. Furthermore, as secondary objective, a schematic suicide risk stratification proposal together with a useful flow-chart for planning suicide preventive and therapeutic interventions has been here proposed for clinicians working in the field of Mental Health, particularly with those subjects affected with mood disorders at higher suicidal risk.
MATERIAL AND METHODS
Search sources and strategies
A broad overview has been here conducted with literature searches performed by using PubMed/Medline. We combined the search strategy of free text terms and exploded MESH headings for the topics of ‘Major Depressive Disorder’ and ‘Suicide’ as following: ((suicide [Title/Abstract]) AND (major depressive disorder [Title/Abstract])). All articles published in English have been properly selected and screened. Studies published through May 31, 2019 were here considered. In addition, secondary searches were performed using the reference listing of all eligible as well as relevant articles and consultation with experts in the field and or manual search.
Study selection, data extraction and management
We considered studies evaluating the Suicide in MDD, by excluding other mental disorders in comorbidity, including anxiety disorders and/or bipolar disorder and/or psychotic disorders. We examined all titles and abstracts, and obtained full texts of potentially relevant papers. After this first screening, we followed a two-step process: 1) in a first phase, we specifically selected all papers containing relevant data on suicide risk factors in MDD subjects (with the aim to identify which suicide risk factors better investigate in the next step, with the aim to address those aspects useful for preventive strategies); 2) in the second phase, we specifically selected a range of macro-categories to be better deepened, as follows: 1) The role of genetic vulnerability and epigenetic modulation in determining suicide risk in MDD subjects; 2) the role of HPA axis in suicide risk in MDD subjects; 3) the role of serotoninergic system in suicide risk in MDD subjects; 4) the role of neurotrophins and neuroplasticity in suicide risk in MDD subjects; 5) the role of neuro-immunological mediators/ biomarkers in suicide risk in MDD subjects; 6) the role of metabolic factors (i.e., lipid profile) in suicide risk in MDD subjects; 7) the role of cognitive domains and neuropsychological dimensions in suicide risk in MDD subjects; 8) the role of personality traits in suicide risk in MDD subjects; 9) evidences coming from neuroimaging studies in suicide risk in MDD subjects. Working independently and in duplicate, two reviewers (LO and DDB) read the papers and determined whether they met inclusion criteria. LO and DDB, independently extracted the data on the above subcategories and selected relevant data useful for the present overview. Disagreements were resolved by discussion and consensus with a third member of the team (FV). All English-language articles identified by the data sources, reporting data on suicide in MDD, both from a preclinical and clinical perspective, have been considered for the present overview. Data collected were then summarized according to the abovementioned categories.
RESULTS
Risk factors
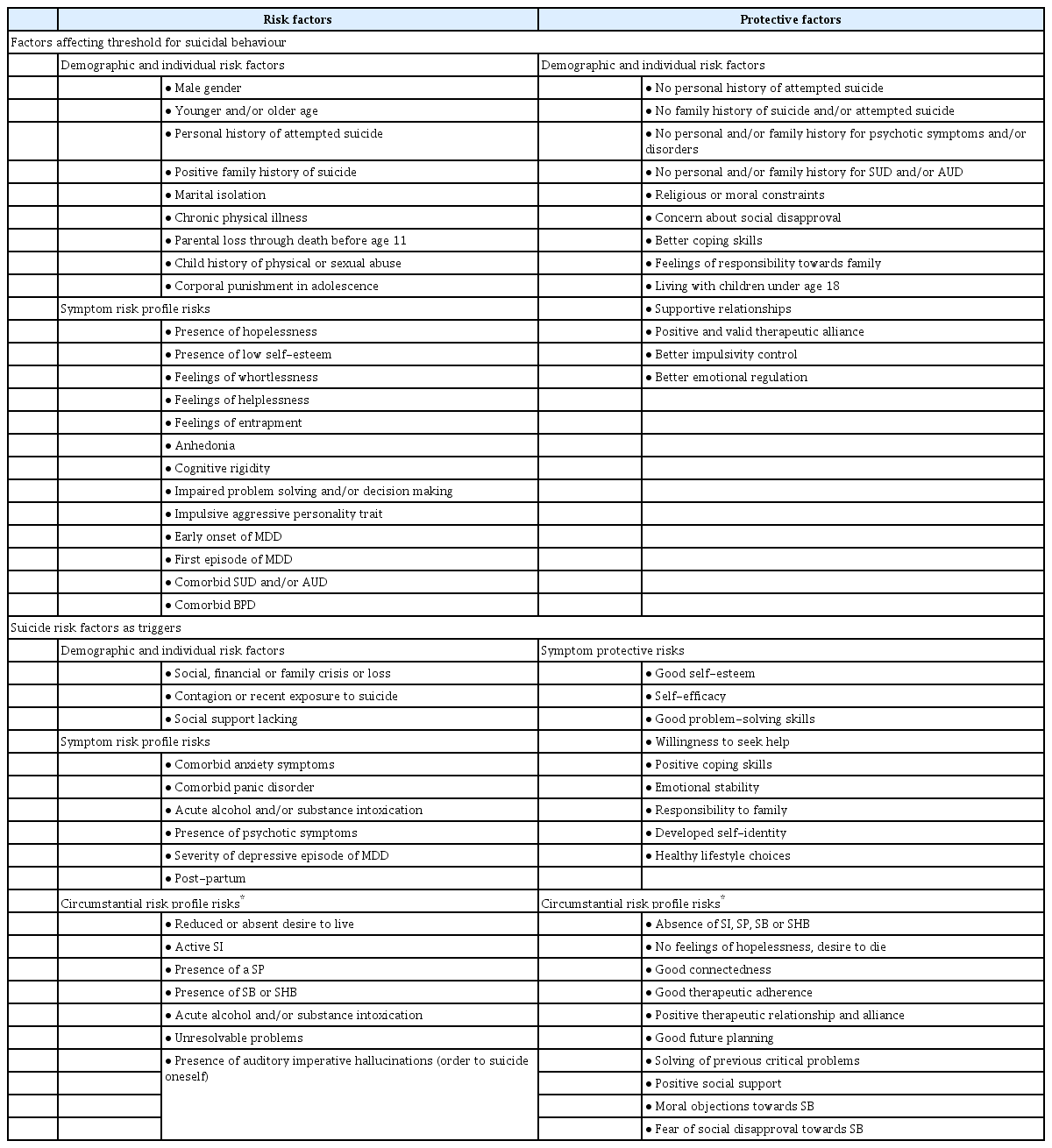
Although the aetiology of suicide and MDD is certainly complex, some suicide risks factors are thought to contribute to the risk of suicidal behaviour, including biological/individual, psychological social, clinical/symptomatological and environmental factors (Table 2) [3,14,17,18].
Genetic vulnerability and epigenetic modulation
Family studies suggest that SA and fulfilled suicide show familial accumulation [19], with heritability estimates of suicidal behavior between 30% and 55% and an increased risk of at least two-fold [20-23]. Inherited genetic differences have a relevant role in suicidality, as demonstrated by twin studies, particularly that monozygotic twins’ concordance for the CS is notably higher than in dizygotic twin pairs, being respectively 24.1% and 2.8% [23-25]. A genome-wide association study (GWAS) of suicidal thoughts and behaviour in MDD, indicated a polygenetic architecture with multiple genes implicated even though with small effects [26]. However, although several GWAS studies have been conducted on SA examining individuals with MDD, comparing suicide attempters with non-attempters and testing for genetic variants that might contribute independently to SA [27-31], epidemiological evidence suggests that the inheritance of suicidality is likely to be independent of the underlying MDD, by supporting a distinct genetic contribution to suicidality [32]. Polygenic risk scores for SA have shown modest predictive capability in independent samples, and small but significant single-nucleotide polymorphism (SNP) heritability estimates for SA have been reported [28,33,34]. A significant association between two SNPs (rs12415800 and rs4746720 in 3’UTR) and CS amongst MDD women aged more than 50 years compared to healthy controls [35]. The FKBP5 gene which encodes the FK506 binding protein 51 (FKBP51) and participates as regulator of the glucocorticoid receptor (GR) activity, has received an increasing attention as well, in relation to the suicidal behaviour [36,37]. FKBP51 is an important modulator of stress response [38]. FKBP5 SNPs have been associated with an increased risk of MDD and SA [39-43]. Amongst the serotonin system candidate genes for SB, many genetic association studies have focused on the SLC6A4 (Solute Carrier Family 6, Member 4) gene [44,45], located on chromosome 17 (17q11.2) which encodes for the serotonin transporter, a transmembrane presynaptic protein involved in the reuptake of the released serotonin from the synaptic cleft [46]. Moreover, the transcriptional activity of SLC6A4 gene is modulated by a 44 base-pair insertion/ deletion polymorphism, commonly known as 5-HTTLPR (serotonin transporter linked polymorphic region polymorphism-rs4795541), located upstream of the transcription start site. Genetic studies demonstrated that depressed suicide victims had a smaller amount of serotonin transporters in the PFC, hypothalamus and brainstem compared to not suicide MDD subjects [47]. Moreover, a recent GWAS study identified GWS SNPs in proximity to genes involved in the regulation of circadian clock rhythms (ARNTL2), anaerobic energy production (LDHB) and catecholamine catabolism (FAH), amongst MDD patients with SA [48]. Therefore, further studies should better evaluate which is common (if any) genetic load in MDD subjects at risk for SA and/or SC and the correlation (if any) is dependent or independent.
Furthermore, distal (predisposing) factors interact with proximal (precipitating) factors in determining suicidal event, i.e., genetic predisposition/vulnerability, early adversities and associated epigenetic modifications, and together may modulate suicidal behaviour and personality traits associated to suicide in MDD [49]. Early life adversity is considered one of the strongest risk factor for SA, i.e., exposure to maltreatment during the early phases of a person’s development increases the risk of SB thought the lifespan within 2- to 5-fold times [50]. In fact, these events may epigenetically regulate key emotional and behavioural systems which in turns may contribute to the development of MDD and suicide behaviour, mainly by inducing a DNA methylation [51-54]. A study investigated whether epigenetic modifications of stress-related genes play a role in suicidal behaviour and whether these modifications are common to or independent of MDD, by reporting a significant increase I DNA methylation of stressrelated genes including BDNF, NR3C1, FKBP5, and CRHBP amongst MDD subjects (with and without SI) compared to healthy controls, together with a concomitant decrease in expression of BDNF, NR3C1, and FKBP5 transcript variant 1, 2 and 3 (but not 4) amongst MDD-suicide subjects compared to healthy controls [54].
The hypothalamic-pituitary-adrenal axis
The HPA axis is the major neuroendocrine system involved in the regulation of the body’s response to stress [1. The stressrelated theory of MDD states that chronic stress may lead to long-term activation of the HPA axis, which may result in reductions in the volume or impaired function of the hippocampus [55]. The corticotrophin-releasing hormone (CRH) and vasopressin are hormones released from neurosecretory nerve terminals and act synergistically to stimulate the secretion of stored adrenocorticotrophic hormone (ACTH) from corticotrophin cells, which in turns stimulates biosynthesis of corticosteroids [14]. Prospective biological studies suggest that dysfunctions in the HPA axis have some predictive power for suicide in MDD [14]. Subjects affected with MDD who manifest a suicidal behaviour show an increased level of CRH in the cerebrospinal fluid compared to not suicide MDD subjects [56]. Several studies reported that cortisol non-suppression in response to the dexamethasone challenge test represents a strong predictor of suicidal behaviour in MDD [14,57-60].
Serotonergic system
The serotonin system has been widely investigated in studies of both MDD subjects and suicidal behaviour. Postmortem studies of the suicidal brains have shown evidence of serotonin dysfunction amongst MDD subjects [61-63]. Serotonin transporters have been reported to be reduced in the prefrontal cortices, hypothalamic, occipital cortices, and brainstems of subjects affected with MDD who have committed suicide [45]. Studies demonstrated a lower concentration of the serotonin metabolite 5-hydryndolacetic acid (5-HIAA) in the cerebrospinal fluid of depressed patients prone to develop suicidal behaviour [64] as well as lower levels of serotonin (5-HT) and 5-HIAA in the brainstem of suicide victims [65-67], compared to non-suicide MDD subjects. The serotonin transporter (5-HTT) is the major determinant of 5-HT inactivation following 5-HT release at synapses, a decrease in 5-HTT has been observed in suicide victims with MDD [68]. Some postmortem studies have reported increased 5-HT2A receptor binding in the prefrontal cortex of suicidal individuals with MDD [69,70]. A meta-analysis of prospective biological studies estimated the odds ratio for the prediction of suicide completion to be 4.5-fold greater for MDD subjects with low levels of 5-HIAA in the cerebrospinal fluid compared to subjects with high levels of 5-HIAA [71]. The serotonin transporter gene is located on chromosome 17q11.1-q12 and two polymorphisms have been reported [72]. Electroencephalographic (EEG) changes and various polysomnographic findings can reflect the central serotonin activity and demonstrate that high suicidality score has been associated with shorter REM (rapid eye movement) latency [73]. An increase of REM time and activity in MDD subjects with SA has been associated with reduced serotonin activity or 5-HIAA levels in cerebrospinal fluid [74].
Neuroplasticity, brain-derived neurotrophic factor and nerve growth factor
Several theories have been proposed to explain the biological substrates of suicide behaviour, including the role of specific neurotrophins, such as the brain-derived neurotrophic factor (BDNF) and the nerve growth factor (NGF) [75-80]. The “neurotrophic hypothesis” of MDD seeks to understand depression through regulatory proteins (e.g., BDNF) which promote neuroplasticity, adult neurogenesis and neurotransmission [81-83]. Changes in brain structures and function, such as reduced neuronal cell numbers, density and size, as well as decreased cortical thickness and changes in synaptic circuits, may be associated with MDD, stress and suicidal behaviour [84,85]. In fact, both MDD and suicidal behaviour involve altered neural plasticity, resulting in an abnormal central nervous system response to stressors and environmental outcomes [79]. The neurotrophic factors activate the neuroendocrine cells and the neuronal responses, by regulating the growth and proliferation of glial cells, modulating the activity of endogenous opioid peptides, activating the HPA axis, exerting effects on corticotrophin releasing hormone-producing neurons, and acting on the endothelial cells of the cerebral vasculature or on the glial cells in the circumventricular organs [86]. Furthermore, the neurotrophic factors may influence as well as the metabolism of the noradrenergic, serotonergic and dopaminergic systems [87]. It has been as well supposed that low BDNF levels relate to suicidality rather than to MDD specifically [88], even though low BDNF levels have been reported in MDD subjects who attempted suicide when compared to non-suicidal MDD and healthy controls [77,80,89]. A recent study found that serum BDNF levels were significantly lower in MDD with SI compared to MDD without SI but were not significantly correlated with MDD severity [90]. CREB1 (cyclic adenosine monophosphate response element binding protein) is a transcription factor that controls the transcription of numerous neuronally expressed genes such as BDNF [91-95]. There is evidence for CREB1 playing an important role in the neurobiology of suicidal behaviour [96-99]. Nerve growth factor (NGF) is a neurotrophin, produced in the cortex, hippocampus and hypothalamus as well as in the peripheral nervous system and immune system [100]. Neurotrophins generally are implicated in neuronal survival, differentiation, connectivity and plasticity during development and adulthood [101]. Clinical studies have detected reduced levels of NGF in patients with MDD and suicide victims, particularly in the prefrontal cortex and the hippocampus [101-104], areas implicated in the cognition and mood regulation as well as in the pathophysiology of affective disorders and suicide [102]. Furthermore, the hippocampus is an area affected by early stress, which in turns is implicated in the suicidal behaviour [101]. However, studies specifically investigating NGF, MDD and suicide risk are scarce and extremely heterogeneous from a methodological point of view, hence, further studies should be carried out in order to better clarify the potential role of NGF in increasing suicide risk amongst MDD subjects.
Neuro-immunological markers
Inflammatory mediators and oxidative stress leading to excitotoxicity may play a critical role in the pathophysiology of MDD and suicide, including an imbalance between proinflammatory cytokines (i.e., interleukin IL-1b, IL-2, IL-6, interferon-gamma INF-γ) and tumor necrosis factor-alpha (TNF-α) versus anti-inflammatory cytokines (i.e., IL-4 and IL-10); or increased levels of pro-inflammatory cytokines and level of severity of MDD [105]. Therefore, a dysregulation of immune response could be a contributing factor to MDD at risk of suicide, including the vascular endothelial growth factor (VEGF) and kynurenine levels [105,106]. Moreover, several findings suggest that suicidal MDD patients display a distinct peripheral blood cytokine profile compared to non-suicidal patients with MDD, being specific changes in inflammatory cytokines levels most frequently associated with MDD and suicidality [105]. In particularly, lower IL-8 levels linked to a reduced neuroprotection and higher IL-13 levels have been found in MDD patients with SI compared to those MDD subjects without [105]; whilst increased levels of interferon-gamma (INF-γ) and IL-6 appeared to be more robustly associated with SA in MDD [107], even though previous studies evaluating suicidal MDD patients reported decreased levels of INF-γ and IL-6, compared with non-suicidal MDD patients [108,109]. Indeed, other studies are quite conflicting in findings, for instance, high IL-4 levels have been found in MDD women with CS, suicidal MDD patients and both suicidal and nonsuicidal MDD subjects [105]. Suicidal MDD subjects, particularly those who were violent SA, were are likely to own higher IL-6 and lower IL-2 levels compared to non-suicidal MDD subjects and healthy controls [105]. Furthermore, higher TNF-α levels have been reported as well in suicidal MDD subjects compared to non-suicidal MDD and healthy controls, even though some evidence appear to be contrasting [105]. A key biological pathway which may link inflammation and MDD is the activation of the HPA axis by cytokines, mainly due to psychosocial stressors, resulting in increased cortisol levels and release of monoamines which may initially enhance inflammatory signaling pathways and active immune system. However, not all studies demonstrated a positive correlation between inflammatory cytokines and suicidal behaviour in MDD subjects, hence, further studies should better investigate this correlation (if any). For a more complete overview, see Marini et al [110].
Metabolic pattern
Large randomized clinical trials of cholesterol-lowering drugs and meta-analytic studies reported an increase in violence-related deaths, including suicide, amongst individuals taking serum cholesterol-lowering medications [111,112]. Suicidal MDD patients tend to have dysregulated lipid levels compared with non-suicidal patients [113-115]. Clinical studies carried out on psychiatric subjects, including MDD subjects, revealed a relationship between lower total cholesterol levels and suicidal behaviour [116-120]. Lower levels of total cholesterol in MDD patients with SI, compared to non-suicide MDD subjects, have been reported in a recent meta-analysis [121]. Low triglycerides, low levels of low-density lipoprotein (LDL) and low levels of high-density lipoprotein (HDL) are significantly related to suicidality in MDD patients [122,123]. A proposed hypothesis suggested that reduced cholesterol levels may reduce serotonin precursors and modify the functions and viscosity of serotonin receptors and transporters, by increasing one’s tendencies towards impulsive, aggressive, and suicidal behaviour. Low serum triglycerides concentrations may also alter serotonin metabolism, leading to poor control of aggressive impulses in MDD subjects, by resulting in an increased suicidality risk [124]. Another hypothesis state that low peripheral and central cholesterol can reduce lipid viscosity of neuronal cell membranes, which may decrease exposures of pre-synaptic serotonin transporter or post-synaptic serotonin receptors [125]. Similarly, further studies should better investigate the role of metabolic (including lipid) profile in determining an increased suicide risk amongst MDD patients, and evaluate how anti-cholesterol and anti-dyslipidemia drugs may reduce suicidality in MDD patients.
Neuropsychological and neurocognitive factors
Patients with MDD show cognitive deficits in neuropsychological domains, such as visual and verbal memory, working memory, attention, executive function and processing speed [126], being the executive functioning impairment the most prominent [127-130]. More specifically, impairments in cognitive control (i.e., the ability to regulate one’s own thoughts and actions in order to achieve internal goals and allows flexible adaptation of behaviour to changing environments), has been strongly associated with MDD-related pathology [131-133]. Impaired cognitive control abilities have been correlated as well with high suicide rate amongst MDD subjects [128,132-137]. In fact, neurocognitive deficits are presumed to increase suicide risk as they may determine an incorrect appraisal of one’s life situation and an impaired decision-making [133,136]. One of the neuropsychological domains strongly impaired in MDD regards the executive function, a set of self-regulatory cognitive processes essential for adaptive behaviour [137-143].
Temperament, character and personality traits
The suicide risk factors implicated in MDD subjects may include distal factors (i.e., those risk factors not strictly related to current episode), such as family history of suicide, early onset of mood disorders, alcohol/substance abuse, adverse early life events, and specific personality traits; as well as proximal factors (i.e., related to current or past mood episode), including hopelessness levels, impulsiveness, SI, severity of current episode within MDD, and recent life events (Table 2). Overall, personality refers to individual features in characteristic patterns of thinking, feeling and consequently behaviours and belong to the stress-diathesis model for suicidal behaviour, being significant influencing factors able to discriminate if a suicidal behaviour emerges within a recrudescence of a psychiatric condition or whether is a situation-oriented process [144]. In fact, specific personality traits, temperaments and characters may predispose a subject with MDD to develop a SI and/or SA or SC. According to the Cloninger’s psychobiological model of temperament and character [145], MDD subjects who had recent SA during a depressive episode exerted different personality profile compared to non-suicidal control group [144]. In fact, MDD subjects suicide attempters, showed significantly higher scores on harm avoidance (HA) (i.e., a tendency to respond intensely to signals or aversive stimuli) and significantly lower scores in self-directedness (SD), cooperativeness (CO) and persistence (PS) when compared to the non-suicidal group [144]. HA is highly heritable temperament dimension linked to the serotonergic system which is in turns altered in suicidal behavior, as abovementioned. SD encompasses personality features like responsibility, self-acceptance, effectiveness; hence, low SD levels have been associated with immaturity, poor self-integration, ineffectiveness and destructiveness which are related to suicidality [145]. Similarly, the alexithymia construct applied to MDD subjects, seemed to demonstrate a correlation between alexithymia traits, MDD severity and increased risk of SI and more severe SA [146,147].
Neuroimaging studies
Neuroimaging studies show changes in several brain areas associated with an increased vulnerability to suicidality [148]. It has been documented that brain dysfunctions located in temporal, parietal and frontal (specifically dorsolateral and orbitofrontal areas) cortices are described in the suicidal brain [148-154]. Moreover, three structural areas, e.g., the left superior temporal gyrus, rectal gyrus, caudate nucleus; and three functional areas, e.g., right cingulate gyrus, the anterior cingulate and posterior cingulate have been identified as implicated in an increased suicidal vulnerability [151,155-157]. A smaller volume of the orbitofrontal cortex (right and left), lower left ventrolateral prefrontal cortex (VLPFC), frontal and temporal lobe volumes were observed amongst MDD patients with SA [158-160]. Reduced grey matter volumes in the frontal, parietal, temporal, insula cortices, left angular gyrus, lentiform nucleus, midbrain, nucleus accumbens, cerebellum have been reported amongst MDD subjects with SA and have been correlated with higher hopelessness levels and lower social support seeking [161-163]. Right amygdala volumes and lower hippocampal volumes are reported amongst MDD subjects with SA compared to non-suicide attempters [158,160,164]. Studies carried out on a sample of MDD subjects by using functional magnetic resonance (fMRI) reported a greater resting state functional connectivity in the amygdala, a greater amplitude of low-frequency fluctuation (ALFF) in the right superior temporal gyrus, left middle temporal gyrus and left middle occipital gyrus, whilst a lower ALFF in the left superior frontal gyrus, right ventral medial frontal gyrus and left middle frontal gyrus, amongst MDD subject with SA compared to MDD without SA [165-167]. Further fMRI studies described a reduced left lateral orbitofrontal cortex (OFC) and occipital cortex activation during risky choices, a higher activation on the left hippocampal and left middle temporal gyrus, amongst MDD with SA [168-170]. Moreover, a dysfunctional emotion processing neural circuitry has been documented amongst MDD subjects with SA [171]. In addition, PET studies reported a lower 5-HTT binding in the midbrain, but not in the ventral PFC or the anterior cingulate network, reduced SERT binding potential in the midbrain/pons and putamen, amongst MDD with SA [172,173]. A SPECT study found reduced SERT binding in the OFC, temporal areas, midbrain, thalamus, basal ganglia and cerebellum of MDD subjects with SA, which in turns are correlated with an increased impulsivity [174].
DISCUSSION AND CONCLUSION
Suicide behaviour is highly prevalent amongst patients with MDD [11,175], however, depression per se is not a useful tool for a proper understanding of the complexity of suicide, and SI is not a proxy for the diagnosis of MDD [176]. The uniqueness of each patient determines the variability of the threshold for sustaining mental pain, a condition dependent on personal experiences, emotional states and intimate situation experienced from childhood [175,176]. Hence, someone could argue that human sadness, most as a reaction to a loss, grief, somewhat crisis, etc., could share features with MDD even in the absence of a validated psychiatric diagnosis [177,178]. In line with this, the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) states, “Diagnosis of a mental disorder should have clinical utility” but “the diagnosis of a mental disorder is not equivalent to a need for treatment. Need for treatment is a complex clinical decision that takes into consideration symptom severity, symptom salience (e.g., the presence of suicidal ideation), the patient’s distress (mental pain)” and “Clinicians may thus encounter individuals whose symptoms do not meet full criteria for a mental disorder but who demonstrate a clear need for treatment or care. The fact that some individuals do not show all symptoms indicative of a diagnosis should not be used to justify limiting their access to appropriate care [176,179]."
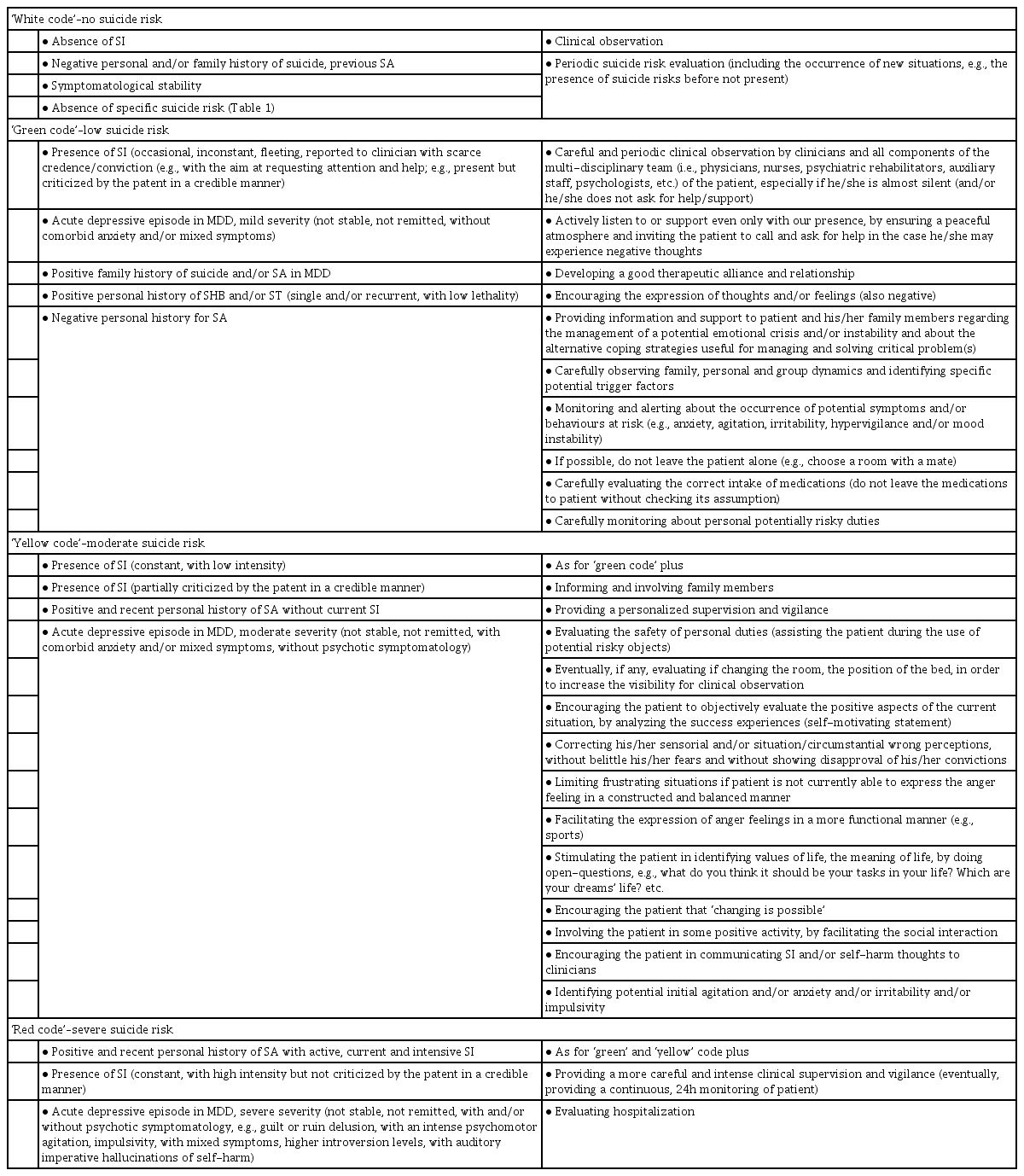
The present comprehensive review aimed at investigating only a selection of the myriad of suicide risk factors supposed to be implicated in the suicidality amongst MDD subjects. Overall, suicidality is indeed a highly complex and multifaceted phenomenon in which a large plethora of mechanisms and processes could be variable implicated, including the dysregulation of HPA activity, genetic load, epigenetics, cholesterol and triglyceride profile, specific neurocognitive and neuropsychological impaired domains, some personality traits and characters, sometimes state-dependent, and so on [8,23,41,43,65,78,110,112,114,128,130,144,145,148,150,175,176]. However, somewhat contrasting and sometimes inconclusive findings have been so far published, by enlarging the plethora of research fields yet to be furtherly deepened and investigated in the field of suicide risk amongst MDD subjects. As abovementioned, great deal of research has focused on dysfunction of the HPA axis and on alterations of main neurotransmitter system as well as a set of neuro-inflammatory modulators, even though concluding findings are still unclear [14,55-60]. Indeed, the recently investigated and interesting role of glutamatergic involvement may play a significant role, given recent antisuicidal findings with NMDA antagonist esketamine [180,181]. Further evidences appear to emerge at the genetic and epigenetic level, with a series of supposed proximal and distal suicide risk factors associated with various endophenotypes implicated in suicidality amongst MDD subjects [19-54]. Therefore, assessing suicidality amongst MDD subjects requires a multidimensional approach, which takes into account suicidality factors at every level, preclinical, neurobiological, neurochemical, clinical and psychopathological. Overall, key suicide and protective risk factors amongst patients with MDD have been clearly recognized and analyzed (Table 2). However, one could argue that SA would be indeed different with CS, regarding a suicide risk stratification as it reflects a different underpinning biological mechanism. Indeed, the most significant predictors of CS appeared to be represented by the presence of a history of previous SA, reaching an odds ratio (OR) of around 4.84 [182]. Therefore, the identification of a range of suicide risk factors, particularly regarding a previous (family and personal) history of SA is clinically relevant for clinicians and should be always considered for preventing CS amongst MDD patients. Beyond these consideration, modern psychiatry needs a better interpretation of suicide risk with a more careful assessment of suicide risk stratification and planning of clinical and treatment interventions, particularly amongst special population [183,184]. Therefore, authors here propose a stratification model of suicide risk accompanied with a list of suggested recommendations regarding interventions and treatments to be planned, useful for clinical practice, particularly for those working in Mental Health (Table 3).
Acknowledgements
None.
Notes
The authors have no potential conflicts of interest to disclose.
Author Contributions
All authors have contributed to the present reviwe with equal efforts.