Fear Network Model in Panic Disorder: The Past and the Future
Article information
Abstract
The core concept for pathophysiology in panic disorder (PD) is the fear network model (FNM). The alterations in FNM might be linked with disturbances in the autonomic nervous system (ANS), which is a common phenomenon in PD. The traditional FNM included the frontal and limbic regions, which were dysregulated in the feedback mechanism for cognitive control of frontal lobe over the primitive response of limbic system. The exaggerated responses of limbic system are also associated with dysregulation in the neurotransmitter system. The neuroimaging studies also corresponded to FNM concept. However, more extended areas of FNM have been discovered in recent imaging studies, such as sensory regions of occipital, parietal cortex and temporal cortex and insula. The insula might integrate the filtered sensory information via thalamus from the visuospatial and other sensory modalities related to occipital, parietal and temporal lobes. In this review article, the traditional and advanced FNM would be discussed. I would also focus on the current evidences of insula, temporal, parietal and occipital lobes in the pathophysiology. In addition, the white matter and functional connectome studies would be reviewed to support the concept of advanced FNM. An emerging dysregulation model of fronto-limbic-insula and temporooccipito-parietal areas might be revealed according to the combined results of recent neuroimaging studies. The future delineation of advanced FNM model can be beneficial from more extensive and advanced studies focusing on the additional sensory regions of occipital, parietal and temporal cortex to confirm the role of advanced FNM in the pathophysiology of PD.
INTRODUCTION
The symptoms of panic disorder (PD) include recurrent panic attacks with the following example symptoms, such as fear of losing control, feel like dying, chest tightness, shortness of breathing, palpitations, dizziness, abdominal discomforts and other physical symptoms with unknown etiology. It is an important anxiety illness but usually under-recognized [1], which is associated with the impairments in life qualities and somatic feelings [2]. PD is easily comorbid with other mental illnesses [3]. PD is also related to diminished well-being, poor sense of health, decline in qualities of life, frequent utilization of medical services, occupational impairments, financial dependency, and marital strife due to recurrent panic attacks and anticipatory anxiety [2,4,5]. The traditional pathophysiology of PD was originated from Gorman’s hypothesis of “fear network model (FNM)” [6]. The FNM included frontal and limbic areas, such as insula, thalamus, periacqueductal gray matter, locus cerulus, parabrachial nucleus and nucleus of solitary tract, medial frontal gyrus, anterior cingulate, amygdala and hippocampus, brainstem, hypothalamus. The frontal areas should modulate the exaggerated fear reactions of the limbic regions properly. Inadequate control of fear response will provoke panic attacks [6]. The subliminal fear is processed via cortical-cortical and cortico-subcortical functional connection [7]. The fear and arousal of panic symptoms are related to attention modulation toward threat band emotional salience of the threat [8]. The center of traditional FNM is the amygdala, which is connected with medial frontal cortex and hippocampus for the control of fear response and fear memory. In addition, the projections from amygdala are linked with brainstem and hypothalamus for the panic symptoms related to the dysfunction of autonomic nervous system. The dysfunction of FNM is usually associated with early childhood life stressors and genetic loading. The serotonin-related medications, such as antidepressants, can relieve the panic symptoms via the decrease in the exaggerated fear response between amygdala and brainstem or hypothalamus. In addition, psychosocial interventions can enhance frontal cortex inhibitory ability for the amygdala and hippocampus [6]. The hippocampus hyperexcitability would enhance the fear memories and the inhibitory neurotransmitter can help the suppression and extinction of fear response related to fear memory [9]. The traditional FNM is associated with the dysregulation of cholecystokinin, serotonin, glutamate, norepinephrine, gamma-aminobutyric acid systems [10,11]. However, recent neuroimaging studies revealed that no such distinct and specific pathways for the medication and psychosocial interventions as Gorman et al. mentioned in their original hypothesis. In addition, recently another review of panic disorder focused on the neuroimaging reports, such as functional, metabolic and structural imaging studies. The review mentioned the hyperactive amygdala should be a state biomarker, not a trait biomarker. The alterations in the cortico-limbic interaction were replicated with further involvements of extended areas, such as anterior cingulate cortex and insula [10,12]. However, the interindividual differences of the FNM for each patient might be associated with inter-individual differences of panic symptoms and clinical responses to treatment [13]. Further advanced FNM should include more potential extension areas. In my viewpoint, the sensory-related brain regions, such as temporal, occipital and parietal should be included in the advanced FNM for the pathophysiology of PD. Since most panic symptoms, such as chest tightness, shortness of breathing, dizziness, palpitations, paresthesia and abdominal pain, etc., are related to sensory function and response. Therefore, this review would reveal the importance of extended areas for the advanced FNM, especially for the sensory regions. I will also review whether the functional connectivity of traditional FNM is associated with sensory-related brain regions and whether the connectivity of sensory-related brain regions will be modulated through thalamus and insula in the following sections.
EXTENDED AREAS OF FNM
The advanced FNM evidences for temporal regions
The model of fear circuitry in PD consists of lateral nucleus of amygdala, hippocampus, frontal cortex, insula, thalamus, anterior cingulate, hypothalamus and brainstem, which are interacted with each other to modulate the panic responses [6,14,15]. For the gray matter (GM), the voxel-based morphometry (VBM) analysis showed an extended region, temporal lobe, which was not included in the traditional FNM [16-22]. Several VBM studies reported alterations in the temporal regions, even with opposite findings of GM volumes, such as decreased GM [17,23] and increased GM in PD patients [22]. The alterations of temporal lobe might be associated with the inhibitory function of frontal cortex. The alterations in the frontal regions of PD patients have been mentioned in several VBM reports [17,20,21,23-26] and the GM volumes were negatively correlated with the severity of PD symptoms [23]. The frontal regions might use “top-down mechanism” to process the sensory messages from temporal regions to control the subsequent panic attacks [27]. In addition to the structural studies, there are some functional studies showing the crucial role of temporal lobe for the pathophysiology of panic disorder. Regional cerebral blood flow was decreased in the superior temporal gyrus and negatively correlated with the panic severity, anxiety level and illness duration [28]. Nash et al. [29] found decreased presynaptic and postsynaptic serotonin 1A receptor bindings in the orbitofrontal cortex and temporal regions of PD patients. The stimulations of panicogenic will also induce panic attacks through decreased ability of inferior frontal cortex to control the panic responses [30].
The sensory-related function has been discussed for the temporal lobe in PD, which included the visuospatial dysfunction and false threat alarm in patients with PD [14,22,31-34]. The impairments in integrating sensory information through the visuospatial system and attention were also reported [22]. In addition, PD patients had deficits to suppress the interference of nonverbal stimuli and reduced verbal cognitive ability to express abstract thoughts [35]. In the traditional FNM, the additional sensory-related structures, such as temporal lobes and parietal lobes, were not crucial areas [6]. However, fear-related acute stressors would activate infero-temporal, temporo-parietal and limbic structures to exchange information between autonomic-neuroendocrine systems and re-orient vigilant attention [36]. The fear conditioning would increase brain activities in the frontal, temporal and parietal lobes [37]. Our antidepressant treatment study in PD also showed increased regional homogeneity of temporal lobe and decreased regional homogeneity of parietal lobe after remission of panic symptoms under antidepressant therapy [38]. Fearful faces stimuli would also induce spatial attention and interact with emotion, which were associated with temporo-parietal negativity and the activities in occipital lobe [39]. The external stressors and related panic attacks would increase brain activities in thalamus and occipito-temporo-parietal regions [40]. The strong and robust activations in superior temporal lobe and several limbic structures would also occur during panic attacks [41]. The increased cerebral activities of superior temporal lobe in PD patients also replicated the above studies [30]. However, different points of view mentioned the opposite phenomenon, such as decreased regional cerebral blood flow in right superior temporal gyrus of PD patients. In addition, regional cerebral blood flows in right superior temporal gyrus were negatively correlated with the duration of illness, severity of clinical anxiety and PD symptoms [28]. However, decreased regional cerebral blood flow in right superior temporal gyrus of PD patients has been reported in another study. In addition, regional cerebral blood flow in right superior temporal gyrus negatively correlated with the duration of illness, severity of clinical anxiety and PD symptoms [28]. The functions of temporal lobe, such as the regulation of anxiety [42], selective aberrant functional connectivity [43], regulation of mood status [42], the involvement of episodic memory and self-projection [43] might be also impaired in PD. Several reports suggested that “fronto-temporoinsula” network might be altered in PD, which also included the temporal lobe [27,30,44]. Antidepressant treatment would also be associated with the increased regional homogeneity (ReHo) of temporal lobe in the remitted patients with PD [38]. The activations in superior temporal gyrus have been observed during panic attacks [41], which also corresponded to hyperfusion of superior temporal gyrus in a positron emission tomography study [45].
Several VBM studies of PD also supported the biomarker characteristics of temporal lobe, such as the GM alterations over this region [17,22,26]. Increased GM volumes have been mentioned in superior temporal gyrus, which might be associated with the dense connection between insula and temporal lobe [22]. However, decreased GM volumes have been observed in temporal lobe [23]. Pure anxiety disorders also had deficits of GM volume in superior temporal gyrus [26], which might work with amygdala and insula to control the panic attacks. In addition, the alterations in the temporal lobe might influence the spread of sensory information to the thalamus for further filtering and subsequent “top-down” regulation of the frontal system [6]. In addition, the fractional amplitude of low frequency fluctuations in the temporal lobe might be dissociated with those in the fronto-parietal lobe [46]. These studies supported that the advanced FNM should include the temporal lobe.
The advanced FNM evidences for insula
Insula might integrate multimodal sensory information due to dense connections with other brain regions, such as frontal and temporal regions. The insula might modulate the panic responses via this fronto-temporo-insula network [27,44,47]. The alterations of GM in insula have been mentioned in several VBM studies in PD [17,22,27,48]. In the traditional FNM, the insula received the thalamus-filtered sensory information and cooperated with frontal regions to control panic attacks [6].
The alterations of insula GM have been mentioned in several VBM studies with opposite findings, such as decreased GM volumes [17,27,49] and increases in GM volume [22,48]. The insula is also a crucial area for the somatic and cognitive pathophysiology of PD [50,51]. The inconsistent findings also occurred in the functional studies of PD patients, such as increased brain activity [48] or decreased brain activity [30]. The typical fear due to danger threat response also decreased the coupling between insula and frontal cortex [52]. The visceral-somatic afferent and efferent impairments were also associated with decreased gamma amino-butyric acid binding in insula of PD patients [53]. The fear of cardiovascular symptoms in PD, response of visual threat, anxiety sensitivity during emotional face processing, anticipating anxiety towards the agoraphobia situation, prediction of cognitive behavioral therapy, pH sensitivity functional imaging and unpredictable aversiveness for avoidance response were also associated with the activations of insula [54-61] in an extended version of fear network [12,62]. The anxiety sensitivity was negatively correlated with white matter (WM) microintegrity [63]. From these literature, insula might play a crucial role for the processing of cognitive, emotional, fearful and primitive response during panic attacks. It should be included in the advanced FNM based on the numerous structural and functional imaging studies.
The advanced FNM evidences for thalamus
The thalamus regulates emotional and cognitive functions, such as fear, arousal, attention modulation towards threat, emotional perseveration of threat, state anxiety for threat monitoring, shock monitoring and sensory processing [8,15,64]. The thalamus interacts with temporal, parietal, subcortical limbic structures, or other parts of fear network structures to modulate the noradrenergic system response towards fear [36,40,64]. It is a part of fear network and can regulate fear response towards threat [6,65]. In addition, our previous study about RFMRI also found alterations of fractional amplitude of low frequency fluctuations in thalamus [66]. Pentazatos et al. [67] reported that fearful face presentation would provoke functional connectivity between AG and hippocampus. In addition, thalamus will connect with temporal lobe and insula for the pathogenesis of PD.
The advanced FNM evidences for parietal lobe
Serotonin-related functional alterations have been found in the parieto-superior temporal regions of PD patients [68]. Another kind of anxiety disorder, social anxiety disorder, had alterations in the neural activities [69] and diffuse impacts on wide resting-state network and selective changes of intrinsic functional connectivity of parietal lobe [43]. The regional cerebral blood flow asymmetry index in temporal and parietal lobes was associated with panic severity [70]. Anxiety-provoking situation also had the phenomenon of decreased cerebral blood flow in parietal lobe [71]. The reductions in regional cerebral blood flows were also observed in the posterior parietal-superior temporal areas of PD patients [68]. The anxiety severity was inversely correlated with the metabolisms in temporal and parietal regions in mood disorder [72].
The GM volume of parietal lobe is associated with anxiety and mood, affective regulation, empathic response, meditation and clamness [42]. Several VBM reports in PD showed reductions in GM volumes of parietal and temporal lobes [17,73]. The decreased cortical thickness in the parietal lobe was also mentioned in PD [74]. The cortical gyrification was also decreased in the parietal and temporal lobes [75].
The parietal-related visuospatial dysfunction might be the component issue for the pathophysiology of PD [76]. The activities of parietal lobule and other advanced FNM regions might predict the treatment response to cognitive behavioral therapy [77], which is replicated in another study [78]. In addition, the antidepressant treatment in remitted patients with PD was associated with the changes of ReHo in the parietal lobe [38]. The study between panic symptom and pH functional imaging study also showed the significant relationship with the parietal lobe, insula and temporal lobe [60]. PD patients also had decreased inter-hemispheric coordination between bilateral parietal lobes [79]. The elevated activities of right parietal lobe occurred while anxious patients performed the task [80].
The precuneus, a part of default mode network, has been reported to be altered in PD [66,81,82], which might have impairments of emotion, somatosensory and self-referential processing functions [83]. In addition, the functional connectivity between anterior cingulate and precuneus was increased in PD, which was associated with the concentration of gamma-aminobutyric acid [84]. An aberrant limbic network between amygdala and precuneus was also found in PD, which might have dysregulation of emotional and somatosensory processing [83]. If the disturbances happened in this network, the emotional and somatosensory processing might be misleading and provoke panic attacks. The alterations in the functional connectivity between dorsal anterior cingulate cortex and precuneus might link anxiety with deficits in self-awareness [85]. In addition, the precuneus-related functions included the attention monitoring, response inhibition, motivation-independent neural process [86], somatosensory processing, emotional processing, visual imagery recall and self-reflection process [87-89], which would be impaired in PD. The decreased parietal activation during avoidance response to affective stimuli was also observed in PD [90]. From the above literature, the parietal lobe should be included in the advanced FNM due to its related sensory function and cognitive function, which corresponds to another review article [12].
The advanced FNM evidences for occipital lobe
Sensory regions of brain, such as occipital or temporal lobe, will transmit the sensory information to FNM for recognizing and processing fear signals of face and body [91]. The fear processing, sensory and inhibitory function might be associated with regional instability of the occipital lobe in PD [81]. The anxious response, sensory-related fear, dysfunctions of self regulation might occur in the occipital lobe of anxiety patients [92]. The anticipatory anxiety, a crucial trait of PD, might increase while higher level of neuroticism. Anticipatory fear was believed to be induced by abnormal brain activities of occipital lobe [93]. In addition, the occipital lobe was associated with face recognition, sensitivity to fearful stimuli, emotion processing and levels of trait anxiety [94,95]. The worry also seemed to decrease regional blood flow in occipital lobe, which confirmed the role of occipital lobe in the worry model for PD [96]. The occipital lobe was related to sensory processing of auditory-spatial and visuospatial information [97,98]. The environmental changes, such as visual or sensory changes, would elicit the responses in the middle occipital gyrus. Sensory changes might precipitate panic attacks, which might suggest that occipital lobe should be a part of FNM [99].
PD is associated disturbances of sensory processing and integration [100]. The limbic system might receive abnormal sensory signals from occipital lobe, which would provoke panic symptoms in the brainstem. The dorsal attention system, which includes the middle occipital gyrus, controls top-down procedure for selective attention and sensory modulation [43]. The connectivity between occipital lobe and other regions would be impaired due to excessive anxiety and fear [101,102]. PD patients had abnormal activities in occipital lobes, basal ganglion and thalamus while receiving negative emotion stimulus [103]. In addition, the 5HT-1A receptor binding potential of occipital lobe was negatively correlated with anxiety levels [104].
In addition to middle occipital gyrus, the visual association cortex (a part of occipital lobe) might be another important part of the FNM. The attention, perception, visual identification, recognition memory, visuospatial ability and interoceptive sensory information processing [105-107] of visual association cortex might be impaired in PD [22]. The shock-related fear might impair the memory consolidation in bilateral lingual gyrus [108], which was associated with significant rapid eye movement sleep and impaired consolidation of fear extinction [108]. The link between amygdala and visual association cortex was responsible for processing fearful faces and spatialrelated information [109]. The results were also in line with altered spatial-related attention due to impaired connection between amygdala and visual area in PD [109]. Visual association cortex was also associated with the regulation of visual imagery and autonomic function [110], sympathetic activity and the processing of autonomic function [111,112], the perceptions of bodily expressions, threatening of fear signals [113], and anticipatory anxiety, 93 which might be impaired in PD. The fear, perception arousal, autonomic dysregulations, and anticipatory anxiety are core symptoms and presentations of PD. Therefore, visual association cortex might be a component of FNM of PD.
We ever reported significant GM deficits in occipital lobe of PD patients who were comorbid with major depressive disorder [20]. Our another report of ReHo in PD also showed alterations in occipital lobe might be a part of FNM [81]. In addition, the impaired fractional amplitude of low frequency fluctuations have been observed in the middle occipital gyrus of PD patients [66]. The lingual gyrus was connected with amygdala to form the FNM for the spatial attention ability and fear-processing in PD [109]. In the advanced FNM, occipital lobe might work with medial structures (such as thalamus and amygdala), frontal regions and other sensory region (such as superior temporal lobe) to process the fear identification and adaptation [114]. The occipital lobe probably send the sensory information through the sensory afferents to the thalamus, amygdala and hippocampus, which are core structures of fear circuitry of PD [6].
The hyperoxic ventilation challenge elevated the activities in occipital lobe. The addition of carbon dioxide abolished the abnormal response of occipital lobe [115]. In addition, panic attacks from the stress of social interaction might be associated with alterations in the occipital lobe for neural processing of social cognition, social rejection sensitivity and low confidence fear [116,117]. The lingual gyrus also regulates the anxiety, vigilance and cardiovascular functions [118]. The visual processing, orientation-specific function of occipital lobe [119], the visuospatial ability, somatosensory stimulation and perception of sensory stimulus [120] suggested that the impairments in sensory integration might predispose to panic attacks. In addition, antidepressant treatment enhanced the brain activities in occipital lobe and relieved the panic attacks [121].
The cuneus, another part of visual association cortex, might also connect with core structures of FNM via sending sensory information to the amygdala, hippocampus and thalamus. The bottom-up control of visuospatial selective attention, which was called stimulus-driven attention, also occurred in the cuneus [122]. The occipital lobe seemed to be interconnected with default mode network and limbic regions for the control of vigilance, attention, motivationand arousal [123]. The occipital lobe was also linked with four cortical networks, such as default mode network, dorsal attention, visual and somatosensory network [124]. The cholecystokinin-4 related model of panic attacks found that brain activations of cuneus were associated with fear scores through the connection with amygdala [41]. Abused women showed abnormal activities of right cuneus and right visual processing regions during response inhibition task [125]. As we know, PD is also usually related to childhood abuse [126] and cuneus might be a crucial part of advanced FNM. In addition, cuneus was responsible for visuospatial attention of threat [127], voice identifications, faces processing and proneness to anxiety reactions [128,129]. In addition, the connectome study of PD showed decreased edge strength of functional connectivity from the right lingual gyrus [130]. The residual alterations in the fractional amplitude of low frequency fluctuations might also occur in the occipital lobe even after antidepressant therapy with remitted status [131]. The occipital lobe-related fasciculus was also altered in the active phase and remitted status of PD patients [132-134]. The inappropriapte attention and sensitivity to the sensory stimulus, such as chest tightness and heart fast beating, might bring the panic attacks. The orienting-avoidance function of cuneus towards threatening events in anxious subjects might be related to avoidance behaviors of panic attacks. The incorrect somatic messages from occipital lobe might provoke the abnormal responses from core structures and send the abnormal responses to the brainstem and other regions to cause the panic attacks. Therefore the occipital lobe should be enrolled as a crucial component of advanced FNM.
Supporting evidences from white matter study
The current WM study focused on the microintegrity of fasciculus. The sensory-related WM fasciculus, such as fronto-occipital fasciculus (FOF) and uncinate fasciculus (UF), have been mentioned in the studies of anxiety. The FOF is a WM tract interacting with language-related WM fasciculus to connect the occipital lobe and frontal system through the parietal lobe [135]. The frontal lobe is also the component of traditional FNM [6]. The frontal lobe can control amygdala-related fear response, which is related to UF’s connection between frontal lobe and amygdala. In addition, anxiety might be linked with the alterations in the microintegrity of UF, which suggested deficient connectivity of UF between orbitofrontal cortex, amygdala and temporal lobe [136-139].
The limbic areas might connect with other brain region for the processing of fear response, which would be uncontrollable while panic attacks [6]. The fear, arousal and attention toward threat would be modulated by the above fear-related regions. The experiences of panic attacks might also be linked with the emotional salience of the threat [8]. The FOF might be important to input occipital and parietal-related sensory information and send frontal-related inhibitory control, which might be crucial for the pathophysiology of PD. The imaging genetics study showed that brain-derived neutrophic factor genotype would be associated with WM development of FOF, which was related to cognitive and intellectual function [140]. The serotonin-related antidepressant would increase neurotrophic factor release in frontal regions, which might support the role of FOF in the WM pathophysiology of PD. In our study, we found antidepressant therapy increased the microintegrity of right UF and left FOF. In addition, the remitted patients with PD might compensate residual WM alterations of right UF through enhancing WM micro-structural integrity of left FOF [132,141]. The antidepressant therapy might increase microintegrity through increasing neurotrophic factor release and relief of oxidative stress [142] in right UF and left FOF. The antidepressant might influence the microstructure of WM tracts via the astrocyte-related factors, such as increasing glucose utilizations, expressions of astrocyte-derived neutrophic factors and lactate release [143], elevated cerebral blood flow and facilitating neurotransmission in neural circuits [144]. The microintegrity of frontolimbic WM tracts was also associated with serotonin genotype polymorphism [145]. The neurotrophic factor would have effects on brain fiber integrity [146]. It also gives us a hint that antidepressant might increase serotonin release, which might modulate WM micro-structural integrity in the left FOF and right UF. These WM structural reports confirmed the role of occipital and temporal lobe in the advanced FNM.
SUPPORTING EVIDENCES FROM CONNECTOME STUDY
The functional connectome study of PD can also support the concept of advanced FNM. Recently our report showed that the alterations in the network including sensory and motor regions, which were connected with parahippocampus central hub [130]. The alterations of parahippocamopus has been mentioned in PD, such as the elevated cerebral blood flow, reduced GM volume and increased benzodiazepine receptor bindings [19,30,147]. Our VBM study found that PD comorbid with depression would parahippocampal GM reductions [20], which corresponded to the meta-analysis results [148]. Two antidepressant studies in PD also demonstrated that antidepressant treatment would increase glucose metabolism in limbic regions, which also included the parahippocampus [149,150]. Our results in left parahippocampus and its central hub role in functional connectome probably represented a large-scale neurophysiological alteration in PD. The altered connectivity between parahippocampus and dorsal cingulate cortex probably suggested the central role of limbic system. Our functional connectome results also corresponded to the lower activities [151] and treatment response-related influences of neuronal stability in dorsal cingulate cortex [152] of PD patients.
Our functional connectome study of PD also showed that the sensory regions, such as occipital lobe (calcarine gyrus and lingual gyrus) and parietal lobe (supramarginal gyrus, SMG), might be altered and influenced by the limbic system [130]. The hyperperfusion of cerebral blood flow in parietal and temporal lobes have been reported in PD [45]. In addition, the brain activities in parietal and temporal lobes were related to the psychopathology severity of PD [153], which were in line with our results of decreased connectivity strength between left parahippocampus gyrus and SMG. The PD patients had decreased activities in occipital lobe and other visual areas while exposing to the face task [154]. The calcarine gyrus is an area of visuosensory function and the terminus of nervous impulses generated in the retina and the following visual response [155]. The lower activities in the LING of PD patients were also discovered while face task [156]. The spatial scene memory and allocentric coding might be disturbed in PD [157]. The alterations within the limbic-sensory network might suggest that impaired control and feedback abilities, which be linked with the panic response while experiencing environmental stressors.
CONCLUSION
According to the above literature, we proposed the advanced FNM as an emerging model of fronto-limbic dysregulation with insula and sensory regions in the temporo-occipito-parietal lobe might be revealed according to the results of recent neuroimaging studies. The sensory regions, such as temporal, occipital and parietal lobes would input the sensory information to the thalamus for filtering the information. Then the filtered information would be integrated at the insula and then sent to the frontal regions for cognitive processing and limbic system for primitive response, which would be imbalanced in PD (Figure 1). The future delineation of advanced FNM model can be beneficial from more extensive and advanced studies focusing on imaging genetics, machine learning and pattern recognition to confirm the role of advanced FNM in the pathophysiology of PD.
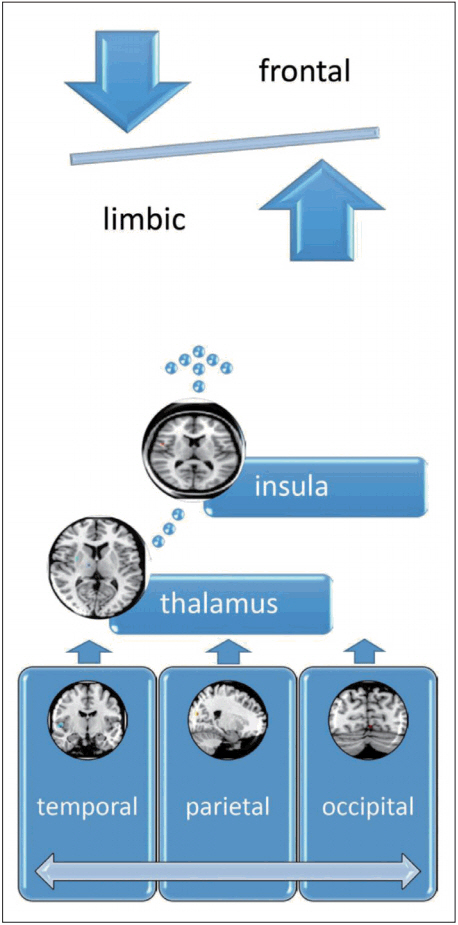
The advanced FNM for PD. The temporal, occipital and parietal lobes would input the sensory information to the thalamus for filtering the information. Then the filtered information would be integrated at the insula and then sent to the frontal regions for cognitive processing and limbic system for primitive response. The extended regions of FNM include the insula, thalamus, temporal, occipital and parietal lobes. FNM: fear network model, PD: panic disorder.